INTRODUCTION
The husbandry procedures and the handling practice effect on stress and on behaviour in rats. It has been demonstrated that even minimal handling is stressful for laboratory rats. Handling for simple tasks such as cage cleaning may cause changes in behavioral, endocrine, and neural parameters relating to emotional and stress responses in young and aged rats (Huot et al. 2002). From that aspect, repeatedly handling rats prior to a behavioural experiment is a routine practice in order to decrease the physiological and behavioural stress response (Rees and Fleming 2001).
In animals - similarly to the parental care in humans - early life experiences related to maternal care play a catalytic role in later mental and physical health (Hughes 2004). Maternal separation and neonatal handling is a well-characterized model of early life stress and of neuroplasticity in rodents (Vallée 1997). Maternal separation increases rates of anxiety, impairs maternal care and diminishes spatial navigation learning (Moscarello, Justin and Joseph 2013). By contrast, neonatal handling generally alters these behaviours in directions opposite to those reported in maternally separated rodents (Theeuwes 2013). Laboratory rat pups are extremely sensitive to rearing conditions. A large amount of literature has shown that the adult behavioural, neurobiological, immunological and neuroendocrine reactivity is affected by the quality of nursing, neonatal handling, maternal separation, environmental enrichment and social isolation (Squire and Dede 2015). Neonatal handling and mildly stressful stimulation in rodents may positively change their behaviour as they age into adults, since they become less susceptible to stress (Daphna and Turk-Browne 2013). Furthermore, neonatal handling results in having adult rats with decreased rates of emotion-related responses such as defecation, freezing, and/or endocrine responses when exposed to novel stimuli/stressors (Whishaw and Kolb 2009).
The Three Rs principles (Replacement, Reduction and Refinement) originated in the scientific community and constitute a widely accepted cornerstone of policies in animal-based science around the world (Gomes et al. 2005). Refinement refers to methods that minimize the pain, suffering, distress or lasting harm that may be experienced by research animals, and which improve their welfare. Example of refinement is ‘’training’’ animals to cooperate with procedures to minimize any distress. Even though previous research highlights neonatal handling as an essential process for rearing less stressed and emotionally sensitive adult rats, there is very little information about a specific neonatal handling schedule that could be a crucial factor in promoting their welfare.
Among the most crucial early life influences is the interaction between the primary caregiver and the offspring (Annemoon et al. 1994). From this point of view this research necessitates the ‘’training’’ of neonatal rats so as to promote their welfare. Moreover, it wants to investigate if a neonatal handling schedule based on imitating rat maternal behavioural patterns may have a positive effect on the rats’ welfare decreasing their stress responsiveness towards humans and allowing their suitability as pets after their life in the lab.
MATERIAL AND METHODS
Animals
Dark Agouti (DA/OlaHsd) rats were obtained from the animal facility of the Centre of Clinical, Experimental Surgery and Translational Research Biomedical Research Foundation of the Academy of Athens (BRFAA), Athens, Greece. The animals were group housed in cages, placed in a well-ventilated house with optimum conditions (temperature 23 ± 1°C; photoperiod 12 h natural light and 12 h dark; humidity 45-50%) with access to food and water ad libitum. Animal experimentation was approved by the Regional Veterinary Service (Ref, Num: 5521 / October 24, 2018) in accordance with the existing National Law on the protection of animals used for scientific purposes, and with the European Directive 2010/63. This study was absolutely non-invasive and was based only on frequent animal handling and human contact.
Procedures
The researchers initially interacted with four (n=4) female rats (M1, M2, M3 and M4). This interaction (which was based on handling, overhead grip and uplifting) started the first day when they were separated from their mother [Day 21 (D21)]. During the gestation period all rats were interacted with the researchers once a week, for 1 hour in total, until they turned 12 weeks old. Then, all female rats mated with the same male rat and after 5 days they were singly caged (Fernández-Teruel et al. 1997) until they delivered their pups. During that period, the researchers continued to interact with the female rats on a weekly basis.
The Neonatal Handling Schedule
Five hours after M1 and M2 had delivered their pups [n=15 (8 females and 7 males)], the researchers started to interact with/handle them on a daily basis (Raineki et al. 2013) for approximately 1 hour in total, until Day 21 (D21). These pups belong to the experimental group ‘’Group A’’. The daily handling session was carried out in a room different from the animal room, maintaining the temperature at 23 ± 1°C. The handling schedule was administered by the same two researchers in the morning (approximately between 10:00 and 11:00 a.m.), and it consisted of first removing the mother from the litter. Afterwards, the pups were gently and individually handled according to the handling schedule demonstrated in Table 1 for a total period of approximately 3 minutes and then placed into plastic cages (Colorado et al. 2006). The neonatal handling procedure (Table 1; Figure 1) was based on attempting to mimic rat maternal behaviour [Escorihuela, Tobeña and Fernández-Teruel 1995; Ladd et al. 2000; Flecknell 2002). Three minutes later, each pup was individually (and gently) handled and stroked for 3–4 seconds and returned to its homecage. When all the pups were back in their homecage, the mother was returned too.
The pups [n=14 (6 female and 8 males)] who were delivered by M3 and M4, belonged to the control group ‘’Group B’’. The classical neonatal handling procedure, as described in previous studies (Fernández-Teruel et al. 1997), consists of moving the mother from the home cage to a holding cage, and then placing the pups individually into small plastic cages for 3 minutes, without applying any handling schedule. After this period, each pup was individually (and gently) handled and stroked for 3–4 seconds and then returned to its homecage. When all the pups were back in their homecage, the mother was returned too.

Table 1. Comparison of human contact response scores between experimental (Group A, exposed to neonatal handling schedule, N=15) and control (Group B, exposed to gold standard neonatal handling, N=14) rats using Mann-Whitney (U) test. Significant results are marked bold. (D=Day)
Experimental Evaluation
On Day 22 (D22) blinded researchers compared the reactions/responses of both the experimental and control groups for the same set of 14 neonatal handling procedures. This comparison was recorded. A third researcher (blind to the study) filled in a customized form with the scores that each pup achieved for each one of the 14 handling procedures (Table 2). Blinding was used, as to try to eliminate biased results, as to examine the rats' behavior against strangers which is a crucial factor for their suitability as pets.
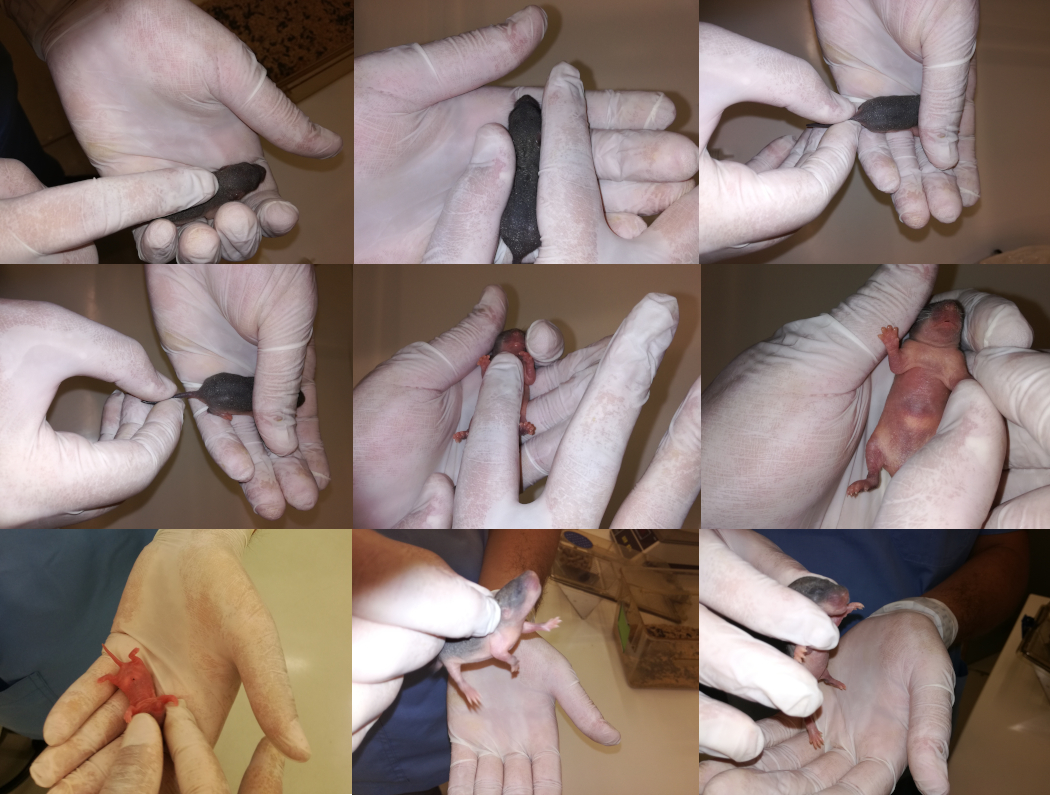
Figure 1. Some neonatal handling procedure. From left to right and top to bottom, n3 holding the rat; n.5 restrain; n.6 touching; n.7 moving the tail; n.9 holding in dorsal position; n.10 holding and touching; n. 11 touching the head; n.12 scruffing; n.13 gripping

Table 2. Scale used to score each behaviour exhibited during the assessment of the 14 handling procedures.
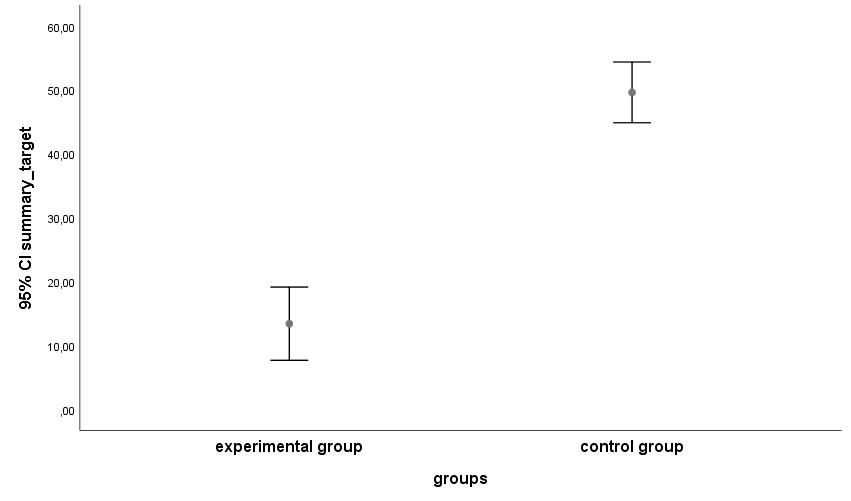
Figure 2. Cumulative response scores to handling procedures by experimental (exposed to neonatal handling schedule, N=15) and control (exposed to gold standard neonatal handling, N=14) rats.
Statistical Analyses
All statistical analyses were performed using IBM© SPSS© Statistics Version 24. The significance level was p < 0.05. The cumulative response scores in reaction to the handling procedures (1-14) at the age of 22 days were compared between experimental (exposed to NH, N=15) and control group rats (no exposure to NH, N=14) using a Mann-Whitney (U) test. Subsequently, the response scores for each handling procedure were separately compared between experimental and control group rats using Mann-Whitney (U) tests.
RESULTS
The cumulative response scores of the experimental group rats were lower than the cumulative response scores of the control group rats (Mann-Whitney (U) test: U = 13; P < 0.01; Figure 2). The rats which had been exposed to neonatal handling in reaction to handling procedures 1 – 14 reacted with lower score responses than those of the control group rats without previous exposure (Table 1).
The experimental group puppies displayed fewer ambulation and cry in the approach handling procedures when compared to the control group rats.
DISCUSSION
Previous studies support the effects of maternal care and how maternal separation may modify both neurobiological and behavioral responses in rodents. This study tested the hypothesis that neonatal handling which imitates rat maternal behaviour could be a crucial factor during the early postnatal period of laboratory rats’ welfare, decreasing their stress responsiveness towards humans and allowing to be adopted as pets.
Humans and nonhuman animals alike have nondeclarative memory consisting of simple associative conditioning, habituation to familiar stimuli, habits and skills which are inaccessible to conscious recollection (Tang et al. 2006). In the unconscious status of nondeclarative memory arise the habits and preferences that are inaccessible to conscious recollection, but nevertheless they are shaped by past events, they influence our current behavior and mental life, and they are a fundamental part of who we are (Mirescu, Peters, and Gould 2004). Study of nondeclarative memory began with motor skills and perceptual skills, but soon included additional abilities such as the phenomenon of priming. Priming is evident as improved access to items that have been recently presented or improved access to associates of those items. In addition, priming improves the speed and efficiency with which organisms interact in a familiar environment and may influence feature-based attentional processes (Pryce and Feldon 2003). Systematic Desensitization appears to be a training technique that is influenced by the phenomenon of priming.
Animals may exhibit, among others, fear behaviours such as freezing (i.e. immobility). However, in a task where learned fear necessitates and is based on an avoidance response (e.g. escape), freezing behaviour is not efficient. In such a case the prefrontal cortex inhibits defense behaviors (e.g. freezing) that are mediated by the amygdala, thereby allowing the animal to escape (Huot et al. 2002). For this reason, the researchers compared the reactions/escape responses of both groups A and B for the same set of 14 neonatal handling procedures (Table 2).
According to the results of this study it appears that those rats that had received handling according to the neonatal handling schedule ‘’Group A’’, were less susceptible to stress during handling (Davidson and Mcewen 2012) compared to those rats that received the gold standard neonatal handling ‘’Group B’’. It seems that neonatal handling schedule beginning on Day 1 (D1) may have significantly affected the rats’ behaviour and decreased the rates of emotional related responses when exposed to handling procedures (Colorado et al. 2006). Group B rats exhibited more intense emotional related responses when exposed to the same handling procedures, which were actually novel stimuli/stressors to them.
One reason why rats are used in scientific studies is to study behaviour since they have such high levels of emotional intelligence. Recent laboratory studies show that rats are highly social animals that depend strongly on conspecific cooperative interactions and consequently show elaborate prosocial behaviours. Well cared for, socialized rats bond readily with their humans. Bonding begins with establishing trust by providing proper care and with hand taming and it continues with continued physical contact and attention. Rather than killing animals that are no longer useful for research, many laboratories have started releasing animals for adoption by personnel and by private homes. Di Gangi et al. (2006) surveyed 458 cats from the University of Florida College of Veterinary Medicine that are no longer used in research and adopted, over a period of six years and found that 91 percent of the animals were still in their original adoption homes, and 80 percent were highly valued family members. It is quite rewarding to watch some of laboratory animals go to good homes after their hard work. With rats and mice, who comprise more than 90% of the animals used for research and testing, there would be many good candidates for adoption. These animals deserve to be in loving homes too. What is needed is for institutions to decide to devote some energy to it. An adjustment to life outside the laboratory can typically be done with ease by applying our proposed handling schedule assessment based on implementation of a neonatal handling procedure that mimics maternal behavior.
The results revealed that a neonatal handling schedule based on imitating rat maternal behavioural patterns may have a positive effect on the rat’s behaviour and reaction during interaction. The schedule of the suggested handling procedures may seem to facilitate and play a crucial role in rats’ welfare and their life after the lab by adopting them as pets. However, further research is needed in order to assess how this positive effect may also affect rats’ stress in later stages (Squire and Dede 2015) and to study how this beneficial impact can also influence the behaviour of rats during adulthood (aged 2½ to 3½ months) and the role of the rat gender, through a series of behavioral tests. In this point of view, further research is needed to study whether neonatal handling based on imitating rat maternal behavioural patterns may also influence behavioral (Social Interaction test, Elevated-Plus Maze test, etc), endocrine (Hypothalamic-Pituitary-Adrenal, Hypothalamic-Pituitary-Gonadal and Somatotropic Axes Activity, etc), and neural parameters (Neuropeptide Expression in the Amygdala and Hippocampus, etc) relating to emotional and stress responses in adult rats (Meijer et al. 2007).
CONCLUSION
The handling is considered a part of the animal’s social environment and plays a crucial role in the animals’ behavior. Proper restraint and handling techniques are essential for reducing stress to laboratory animals and the handler. Animals become much easier to handle if they are trained and accustomed to handling (Rees and Fleming 2001). Although, the proper handling technique has not been standardized. Our study demonstrates the benefits of a standardized handling schedule assessment based on implementation of a neonatal handling procedure that mimics maternal behavior in the rats’ welfare and shows that this handling technique should be the key for continuing their life after the lab, by adopting them as pets.
ACKNOWLEDGEMENTS
The authors would like to thank the husbandry staff of the Animal Facility of Biomedical Research Foundation of the Academy of Athens (BRFAA) for their support, assistance and caretaking. Many thanks also to Dimitra Danai Papageorgiou, Master's in education (M.Ed.) in Teaching English to Speakers of Other Languages (TESOL) for her help.
DISCLOUSURE
An earlier version of this work was presented as an oral presentation at the 14th Pan-Hellenic Veterinary Conference in Thessaloniki in May 2018 (see oral presentation ΠΑ058).
REFERENCES
Colorado, R., Shumake, J., Conejo, N., Gonzalez-Pardo, H., & Gonzalez-Lima, F. (2006). Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behavioural Processes, 71(1), 51-58. doi: 10.1016/j.beproc.2005.09.007
Davidson, R., & McEwen, B. (2012). Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience, 15(5), 689-695. doi: 10.1038/nn.3093
DiGangi, B., Crawford, P., & Levy, J. (2006). Outcome of Cats Adopted From a Biomedical Research Program. Journal Of Applied Animal Welfare Science, 9(2), 143-163. doi: 10.1207/s15327604jaws0902_4
Escorihuela, R., Tobena, A., & Fernandez-Teruel, A. (1995). Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman high-avoidance) and hyperemotional (Roman low-avoidance) rats. Learning & Memory, 2(1), 40-48. doi: 10.1101/lm.2.1.40
Fernández-Teruel, A., Escorihuela, R., Castellano, B., González, B., & Tobeña, A. (1997). Behavior Genetics, 27(6), 513-526. doi: 10.1023/a:1021400830503
Flecknell, Paul. (2002). Replacement, Reduction and Refinement. ALTEX : Alternativen zu Tierexperimenten, 19(2):73-8.
Gomes, C. M., Raineki, C., Ramos de Paula, P., Severino, G. S., Helena, C. V., Anselmo-Franci, J. A., Franci, C. R., Sanvitto, G. L., & Lucion, A. B. (2005). Neonatal handling and reproductive function in female rats. The Journal of endocrinology, 184(2), 435–445. doi: 10.1677/joe.1.05907
Hughes R. N. (2004). The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience and biobehavioral reviews, 28(5), 497–505. doi: 10.1016/j.neubiorev.2004.06.006
Huot, R. L., Plotsky, P. M., Lenox, R. H., & McNamara, R. K. (2002). Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain research, 950(1-2), 52–63. doi: 10.1016/s0006-8993(02)02985-2
Ladd, C. O., Huot, R. L., Thrivikraman, K. V., Nemeroff, C. B., Meaney, M. J., & Plotsky, P. M. (2000). Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in brain research, 122, 81–103. doi: 10.1016/s0079-6123(08)62132-9
Meijer, M. K., Sommer, R., Spruijt, B. M., van Zutphen, L. F., & Baumans, V. (2007). Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Laboratory animals, 41(2), 161–173. doi: 10.1258/002367707780378168
Mirescu, C., Peters, J. D., & Gould, E. (2004). Early life experience alters response of adult neurogenesis to stress. Nature neuroscience, 7(8), 841–846. doi: 10.1038/nn1290
Moscarello, J. M., & LeDoux, J. E. (2013). Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33(9), 3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013
Pryce, C. R., & Feldon, J. (2003). Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neuroscience and biobehavioral reviews, 27(1-2), 57–71. doi: 10.1016/s0149-7634(03)00009-5
Raineki, C., Lutz, M. L., Sebben, V., Ribeiro, R. A., & Lucion, A. B. (2013). Neonatal handling induces deficits in infant mother preference and adult partner preference. Developmental psychobiology, 55(5), 496–507. doi: 10.1002/dev.21053
Rees, S.L., Fleming, A.S. How early maternal separation and juvenile experience with pups affect maternal behavior and emotionality in adult postpartum rats. (2001). Animal Learning & Behavior 29, 221–233. doi: 10.3758/BF03192889
Shohamy, D., & Turk-Browne, N. B. (2013). Mechanisms for widespread hippocampal involvement in cognition. Journal of experimental psychology. General, 142(4), 1159–1170. doi: 10.1037/a0034461
Squire, L. R., & Dede, A. J. (2015). Conscious and unconscious memory systems. Cold Spring Harbor perspectives in biology, 7(3), a021667. doi: 10.1101/cshperspect.a021667
Tang, A. C., Akers, K. G., Reeb, B. C., Romeo, R. D., & McEwen, B. S. (2006). Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proceedings of the National Academy of Sciences of the United States of America, 103(42), 15716–15721. doi: 10.1073/pnas.0607374103
Theeuwes J. (2013). Feature-based attention: it is all bottom-up priming. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 368(1628), 20130055. doi: 10.1098/rstb.2013.0055
Vallée, M., Mayo, W., Dellu, F., Le Moal, M., Simon, H., & Maccari, S. (1997). Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. The Journal of neuroscience : the official journal of the Society for Neuroscience, 17(7), 2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997
van Erp, A. M., Kruk, M. R., Meelis, W., & Willekens-Bramer, D. C. (1994). Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behavioural brain research, 65(1), 47–55. doi: 10.1016/0166-4328(94)90072-8
Whishaw, I. Q., & Kolb, B. (Eds.). (2005). The behavior of the laboratory rat: A handbook with tests. Oxford University Press.